IUD insertion pain addressed in new CDC guidelines
Study finds that teens on birth control pill report more crying, sleeping
On average, researchers found that the 16-year-old participants who were using oral contraceptives reported more crying, sleeping and eating problems than those who weren’t on the pill.
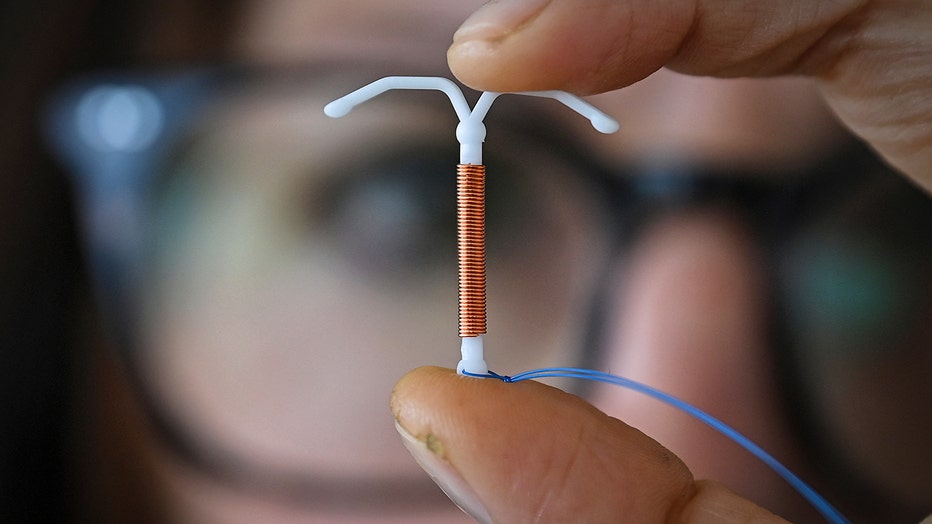
The modern intrauterine device, or IUD, hit the market six decades ago. But in recent years, its reputation for being incredibly painful to place has become infamous on social media.
Now – for the first time ever – the Centers for Disease Control and Prevention is addressing the pain of insertion and offering new guidance for doctors to discuss pain management options.
The report replaces a 2016 report with new and revised recommendations including the provision of medications during IUD placement.
"Before IUD placement, all patients should be counseled on potential pain during placement as well as the risks, benefits, and alternatives of different options for pain management," the guidance reads. "When considering patient pain, it is important to recognize that the experience of pain is individualized and might be influenced by previous experiences including trauma and mental health conditions, such as depression or anxiety."
CDC does not suggest misoprostol for IUD placement
The health agency recommended that misoprostol not be used for routine use in IUD insertions. According to the agency, evidence suggests that the medication does not reduce patient pain nor improve ease of placement or placement success.
FILE: IUD device (Credit: VALENTINE CHAPUIS/AFP via Getty Images)
The CDC also said evidence suggests that misoprostol might actually increase patient pain and other side effects including adbdominal pain, cramping and diarrhea. They said misoprotol might be useful in only select circumstances.
Lidocain recommended for IUD insertion
In its updated guidance, the CDC also recommended that doctors use lidocain to numb the cervix, as evidence suggests that the medication "might reduce patient pain." Lidocain also does not increase side effects like tinnitus, vomiting or dizziness.
RELATED: FDA panel backs Opill over-the-counter birth control
The choices for lidocain now include anesthetic gel, creams and sprays. Experts say the new recommendations expand the options available to doctors.
Currently, 10.4% of women between the ages of 15-49 currently use long-acting reversible contraception such as an Intrauterine device or contraceptive implant.
This story was reported from Los Angeles.

